Monograph Analysis Of Herbal Drugs
American Herbal Pharmacopoeia AHP Chinese drug monographs and analysis Pharmacopoeia of the Peoples Republic of Chin a etc. Who the herbal product is intended for.
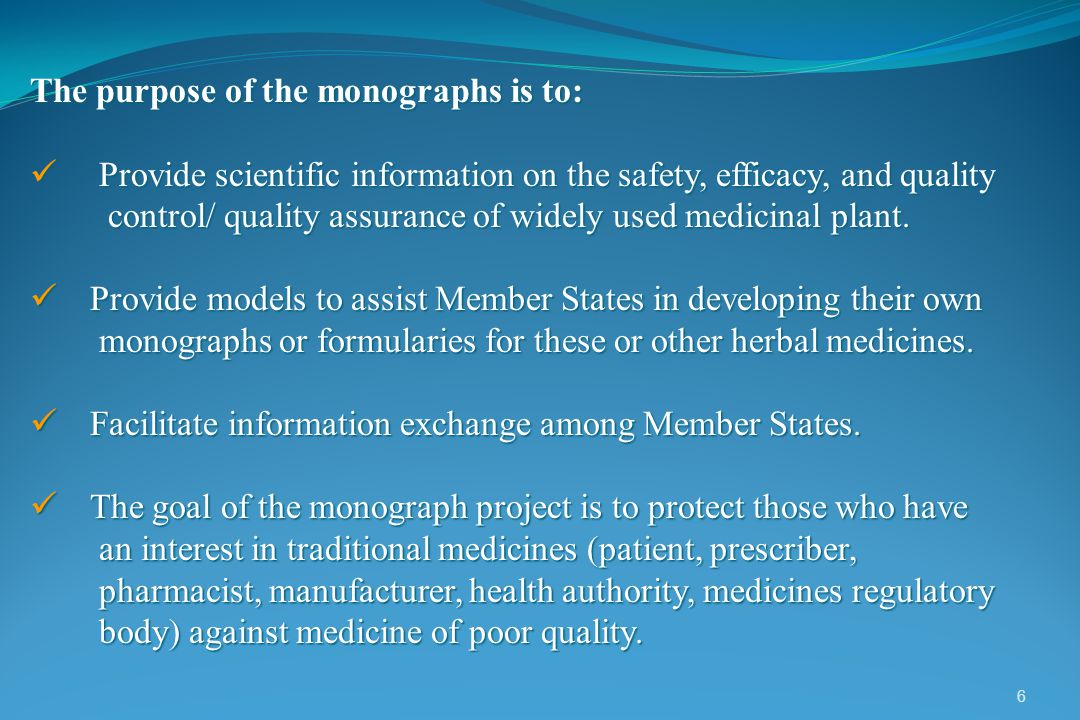
Comparative Study And Importance Of Monographs Of Standards Of Medicinal Plant And Their Parts For Herbal Medicinal Product Prepared By Guided By Ppt Video Online Download
EU monographs provide all information necessary for the use of a medicinal product containing a specific herbal substance or preparation.

Monograph analysis of herbal drugs. Amukkara is the dried root of Withania somnifera L Dunal Syn. The number of monographs on Herbal drug preparations in Ph. Are 156 monographs for herbal drugs and 133 for herbal drug preparations.
The European Medicines Agency Committee on Herbal Medicinal Products HMPC has adopted a Community Herbal monograph for European goldenrod. Two volumes of British herbal compendium vol 1 vol 2. Chromatographic analysis of herbals can be done using TLC HPLC HPTLC GC UV GC-MS fluorimetry etc.
RAGHAV DOGRA BRANCH. Nomenclature for herbal drugs. VIKRAM VARMA 53 54.
63 articles are for galenical preparations tinctures extracts homoeopathic preparations 33 monographs for essential oils and 40 articles on vegetable and animal fats oils and waxes. Monograph on Herbal drugs 1433 should be applied unless otherwise justified. Prepared by British herbal medicine association in 1964.
Acuvakanthi Amukkara. Under traditional use the monograph specifies that it is used to increase the amount of urine as adjuvant in treatment of minor urinary complaints25 The German Commission E has approved European goldenrod. Testing of full Monograph.
Eur which is val-id from the start of 2014 Ph. 23 Types of herbal drugs European Pharmacopoeia contains more than 120 specific monographs about herbal drugs. All tests and assay methods must be validated mainly if the content of constituents individual or mixtures of related.
What the herbal product is used for. Limiters can be utilized as a quantitative or semi quantitative tool to ascertain and control the amount of impurities like the reagents used during abstraction. A vegetal drug which have a plant origin may consist of subteran organs radix rhizoma tubera bulbus bark cortex or aerial organs folium flos fructus pseudofructus.
The Monograph for herbal drugs describes. Introduction Pharmacopoeial monographs for herbal medicines should contain information in the definition that is consistent with the monograph title followed by. Testing of Herbal Medicinal Substances Sampling.
Rather TLC is used as an easier. In this context a monograph is a document that defines a botanical drug and provides information that allows for its proper identification. An herbal monograph is a document that defines a botanical drug and provides information that allows for its proper identification.
BRITISH HERBAL PHARMACOPOEIA 29. Amongst galenical preparations the largest is the number of dry extracts 22 followed by the monographs on. For other metals not listed in this monograph it will ultimately depend on safety considerations.
In this slide contains Introductionnof Indian pharmacopeia ayurvedic unani pharmacopeia and monographs of herbal drugs. Prepared by British herbal medicine association in 1964. Herbal monographs in national pharmacopoeias and other authorative documents play an important role in the authentication of herbal materials.
Upon completion of this course the student should be able to. Individual monographs on herbal drugs and herbal drug preparations General monographs and individual monographs are complementary The drugpreparation described in the individual monograph has to comply with its specific requirements and with the requirements of the general monograph Requirements which are described in the general. Know the WHO and ICH guidelines for evaluation of herbal drugs.
The herbal monographs give a basic description of the herb and list its chemical. This guidance for monographs on herbal medicines has been developed outlining the structure and contents of a herbal medicine monograph. Where justified specifications for sulfated ash residue on ignition and heavy metals should be included and should follow pharmacopoeial requirements.
Form the analysis of ochratoxin A according to EP 2822. Microbiological parameters include total viable content total mold count total enterobacterial and their count. The BHP is a very useful aid to quality assurance particularly for herbs not featured in official pharmacopoeias.
In the VІІIth edition of the Ph. One of the most important aspects for a correct analysis of herbal drugs is the sampling and assessing the. The test procedures included in a monograph should be verified in 2 or more laboratories and the laboratory reports on this verification should be provided to the EDQM to ensure future traceability.
This Guide develops the specific. They find their way into herbal drugs by intake through water and soil as well as through atmospheric sediments. We already have experience in the analysis of herbal drugs for.
1983 edition contain 233 monographs on single herbal drugs and BHP 1996 provide quality standards for 169 herbal raw materials. It contains the basic description including nomenclature part used constituents range of application contraindications and side effects incompatibilities with other medications dosage use and action of the herb. The instructions describing any methodof.
8 2014 the total number of monographs for herbal drugs is 170 and that of herbal drug preparations is 136 of which 63 articles are about galenic herbal drug preparations. 1983 edition contain 233 monograph on single herbal drug and BPH 1996 provided quality. 1 A monograph on a herbal drug or a herbal drug preparation is drafted with the same overall 2 structure as a monograph on a chemical substance and both the latest versions of the 3 Technical guide for the elaboration of monographs and of the Style guide apply to 4 monographs on herbal drugs and herbal drug preparations.
Product analysis and they must follow the rules that even for the mostly very complex composition of a herbal drug any monograph must be drafted following the structure and the rules for a defined chemical substance. Physalis somnifera L P. Understand raw material as source of herbal drugs from cultivation to herbal drug product.
Some Monograph of herbal drugs according to siddha and unani pharmacopoeia 1. Heavy Metals The heavy metals lead cadmium mercury as well as arsenic are omnipresent within our environment not least due to various industrial processes. Know the herbal cosmetics natural sweeteners nutraceuticals.
Safety information such as information regarding undesirable effects and interactions with other medicines. Two volumes of British herbal compendium volume I and volume II. Appreciate patenting of herbal drugs GMP.

Pdf Comparative Analysis Of Monographs On Herbal Drugs And Herbal Drug Preparations Included In The European Pharmacopoeia Ph Eur 8

Pdf Comparative Analysis Of Monographs On Herbal Drugs And Herbal Drug Preparations Included In The European Semantic Scholar

Pdf Comparative Analysis Of Monographs On Herbal Drugs And Herbal Drug Preparations Included In The European Semantic Scholar
Post a Comment for "Monograph Analysis Of Herbal Drugs"